MedTechConsulting
Medical Devices registration
Documentation development
Testing organization
MedTechConsulting LLC has been providing consulting and methodological services for registration of the wide range of medical devices in Russia since 2011
MD of risk classes 1, 2a, 2b, 3
MD for In Vitro diagnostics
Large-sized MD, such as CT, MRI, X-rays etc.
Electrical and non-electrical MD
Disposable and reusable MD
Sterile and non-sterile MD
Implantable and non-implantable MD
We are trusted by many
The foundation of our company’s success lies in our unwavering commitment to an honest and transparent business approach, high-quality work, professionalism, customer-centricity, adaptability amidst constantly changing legislation, and, most importantly, the value of human relationships.
Modern manufacturers of medical devices and equipment of various purposes and complexities are eager to enter the Russian market as quickly and cost-effectively as possible. Our company assists in realizing these objectives, providing manufacturers with the necessary consultations and comprehensive support throughout the process of registering medical devices and in vitro diagnostic products.
OF EXPERIENCE
%
SUCCESSFUL CONTRACTS
CLIENTS
“MedTechConsulting” is dedicated to achieving high standards at every stage of our work, as evidenced by our success in obtaining registration certificates, the high efficiency of our work model, the dynamic growth of our company, and our market reputation.
OUR
COMPANY PROFESSIONAL SERVICES
Expert knowledge in the field of medical devices registration
International work experience with largest foreign and Russian companies.
The consulting firm “MedTechConsulting” provides advisory and methodological services for conducting toxicological, technical, and clinical trials, including clinical laboratory tests, as well as for compliance assessment and the development of necessary regulatory documentation for the registration of medical devices with Roszdravnadzor. The company offers professional assistance in amending registration dossier documents, including carrying out all necessary actions in the event of negative state control conclusions. A distinctive feature of the company is its extensive experience in registering individual in vitro diagnostic medical devices, as well as the registration of devices as part of a “line” (joint testing of analytical systems), particularly analyzers, software, reagents, consumables, and laboratory equipment.
Preparation and request of the documents
Request and analysis of necessary documents, as well as validation whether such documents were prepared and notarized in accordance with the requirements of the legislation of the Russian Federation
Import permission
Organization of obtaining permits for the import of a test sample, organization of on-site tests on the territory of the Russian Federation and abroad (for foreign-made medical devices)
Technical documentation development
Conformity assessment, development and writing of a technical and operational documentation, verification and correction of documents prepared by the Manufacturer.
Organization of testing
- Technical tests;
- EMC tests;
- Toxicological tests;
- Clinical trials, including for in vitro devices
The registration certificate (RC) is a document which confirms the compliance of medical devices with the established requirements and the fact of their registration in Russia.
What is registration and why is it necessary to register medical devices?
Following paragraph 4 of Article 38 of the Federal Law N 323-FZ “On the Basics of Protecting the Health of Citizens in the Russian Federation”:
the circulation of medical devices is allowed on the territory of the Russian Federation only if they were registered in the procedure established by the Government of the Russian Federation, by its authorized federal executive body
Registration of medical devices is a state procedure developed to bring to the market only those products which are effective, safe and have high quality.
In other words, the registration of medical devices is a prerequisite for their import, use, sale, as well as production on the territory of the Russian Federation.
- Until December 31, 2025, you can choose the type of registration of a medical device and register it either according to national (internal rules) or according to the rules of the EAEU.
-
After December 31, 2025, all medical devices will need to be registered according to the unified rules of the EAEU (!Unless the validity of national legislation is extended, as it was extended before).
Registration of medical devices on the territory of the Russian Federation is conducted by the Federal Service for Supervision of Healthcare of the Russian Federation (RosZdravNadzor / RZN)
There are several options for registering medical devices in Russia:

LEGISLATION OF THE EURASIAN ECONOMIC UNION
Makes it possible to circulate medical devices on the territory of all EAEU countries (in case of a positive decision and recognition of the examination conclusion by all EAEU member countries of your choice).
Currently, the EAEU includes five countries: The Republic of Armenia, The Republic of Belarus, The Republic of Kazakhstan, The Kyrgyz Republic, The Russian Federation.
The main legal acts regulating the registration of medical devices in EAEU:
- Agreement on common principles and rules for the circulation of medical devices (medical products and medical equipment) in the framework of the Eurasian Economic Union from 23.12.2014
- Decision of the EEC Council No. 46 “On the Rules for registration and examination of the safety, quality and effectiveness of medical devices”

NATIONAL LEGISLATION OF THE RUSSIAN FEDERATION
Makes it possible to circulate medical devices only on the territory of the Russian Federation.
The main national legal acts regulating the registration of medical devices in Russia:
- Federal Law N 323-FZ “On the Basics of Protecting the Health of Citizens in the Russian Federation”
- Decree of the Government of the Russian Federation N 1416 ” On Approval of the Rules for State Registration of Medical Devices “
- Decree of the Government of the Russian Federation N 552 “On approval of the specifics of circulation, including specifics of state registration, of medical devices in the event of their defectiveness or the risk of defectiveness in connection with the introduction of restrictive measures of an economic nature in relation to the Russian Federation“
- Decree of the Government of the Russian Federation N 430 “On the peculiarities of the circulation of medical devices, including state registration of a series (batch) of a medical device”
Implementation Of A Quality Management System And Production Inspection
- Decree of the Government of the Russian Federation of February 9, 2022 N 136 ‘On approval of requirements for the implementation, maintenance and evaluation of a quality management system for medical devices depending on the potential risk of their use’
- Decree of the Government of the Russian Federation of February 9, 2022 N 135“On approval of the Rules for organizing and conducting inspections of the production of medical devices for compliance with the requirements for the implementation, maintenance and evaluation of the quality management system for medical devices, depending on the potential risk of their use”
Implementation of the quality management system in compliance with ISO 13485 from 01.01.2024:
| Class risk of MD | QMS |
| 1 | voluntarily |
| 2a (non-sterile) | voluntarily |
| 2a (sterile) | obligatorily |
| 2b | obligatorily |
| 3 | obligatorily |
As part of the registration dossier submitted to Roszdravnadzor for state registration from January 1, 2024, a report on the initial inspection of production in compliance with ISO 13485, carried out by the Russian experts of RZN must be submitted.
Medical Devices National Registration Process
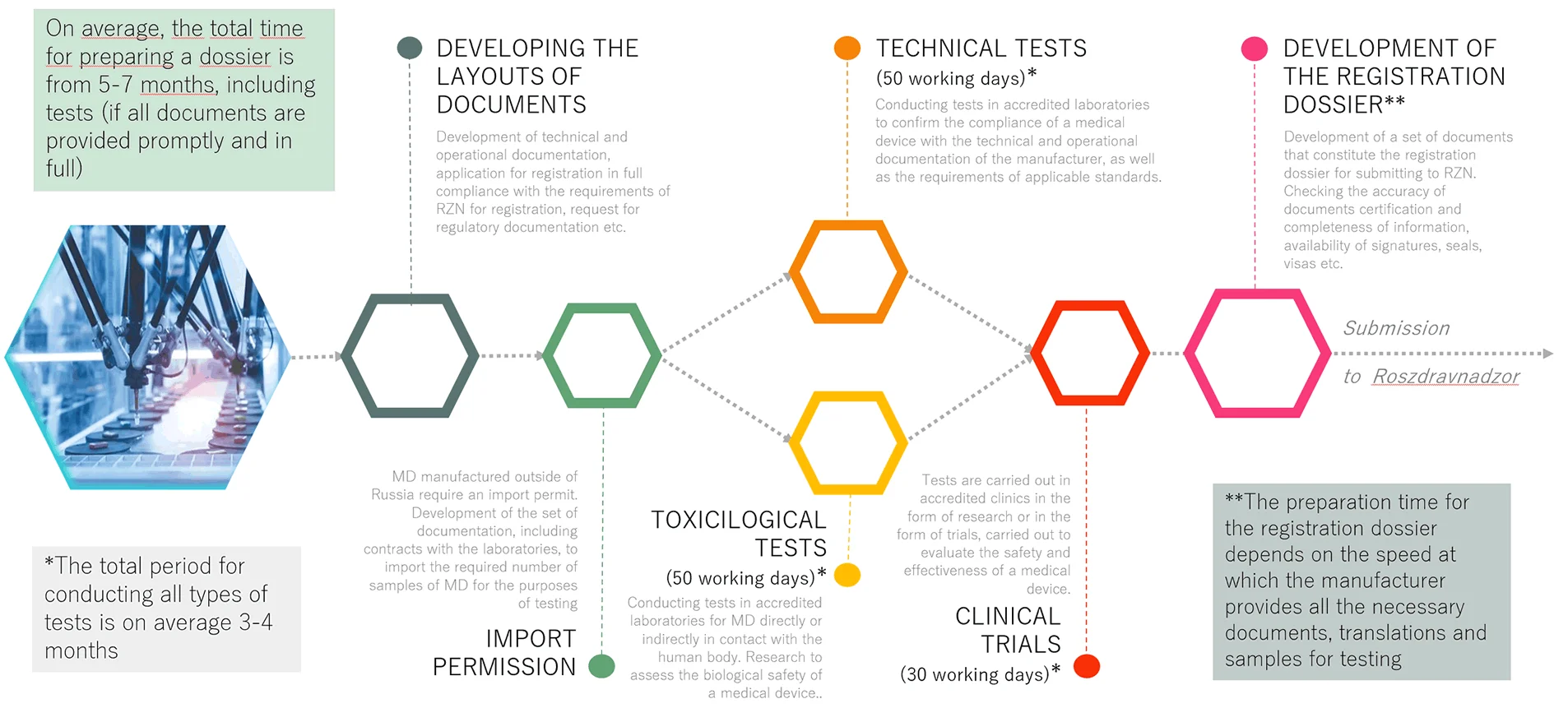
01. PREPARATION OF THE REGISTRATION DOSSIER
Preparation and collection of registration dossier documents; assessment of compliance of the medical device with the declared characteristics and mandatory requirements (technical tests, toxicological tests, clinical trials and other types of tests and research provided for by the current legislation of the Russian Federation)
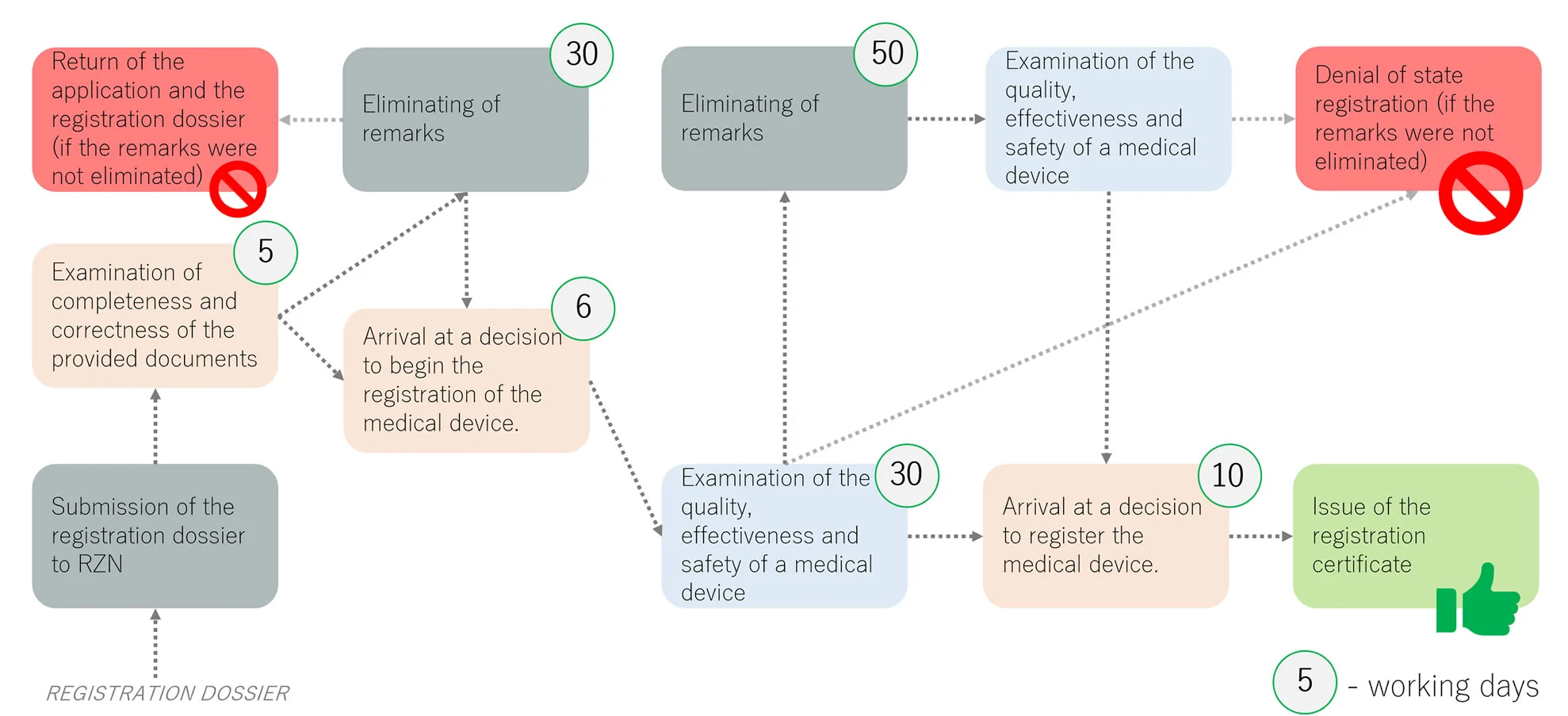
02. REGISTRATION OF MEDICAL DEVICES IN ROSZDRAVNADZOR
Submission of the registration dossier to Roszdravnadzor and examination of the quality, effectiveness and safety of a medical device
Request a Consultation for Medical Device Certification and Registration
Complete our short questionnaire and receive 10% off our consultation services.